Abstract
BACKGROUND : Thrombopoietin receptor agonists (TPO-RAs) are widely utilized second-line treatments for immune thrombocytopenia (ITP). The TPO-RAs eltrombopag and romiplostim have been FDA approved for over a decade with established efficacy and safety profiles.
Avatrombopag is a newer oral TPO-RA approved in 2019 for ITP. Avatrombopag was efficacious in raising platelet counts in clinical trials, and it has an exposure-adjusted safety profile generally comparable to placebo with no boxed warning for hepatotoxicity as does eltrombopag. Also unlike eltrombopag, avatrombopag does not chelate polyvalent cations; therefore, it is administered with food and without restrictions regarding meal composition. A high proportion of patients (~90%) respond to avatrombopag; however, data describing the durability of platelet response on avatrombopag following treatment with other TPO-RAs is limited.
AIMS : Understand the time until patients treated with avatrombopag experienced their first loss of response, if any, and their percent of time with a response following switch from eltrombopag or romiplostim.
METHODS : We retrospectively evaluated all adults with ITP who switched from eltrombopag or romiplostim to avatrombopag at four U.S. tertiary ITP referral centers from July 2019 through December 2020. Reason for switching from eltrombopag or romiplostim (ineffectiveness, adverse event, convenience) was collected. Patients were treated with avatrombopag for at least two months to evaluate effectiveness. Response was defined as a platelet count ≥30,000/uL. Loss of response was defined as two consecutive platelet counts at least 7 days apart <30,000/uL. In these analyses, platelet counts were disqualified if <8 weeks from receipt of rescue corticosteroids or <4 weeks from intravenous immunoglobulin.
RESULTS: 44 patients were included, with a median (range) age of 60 (21-87) years; 55% were female. At avatrombopag initiation, patients had an ITP diagnosis for a mean of 8.1 years with a mean of 4.8 unique prior ITP therapies.
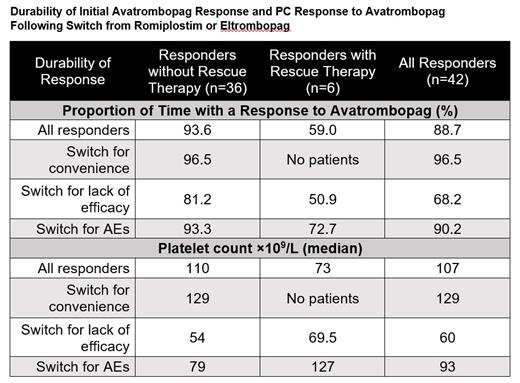
42/44 (95%) of patients responded to avatrombopag at least once and 36/44 (81.8%) responded without the need for rescue therapy. 6/44 (13.6%) responded to avatrombopag and required at least one rescue therapy during exposure.
31/42 (73.8%) of patients never experienced a loss of response. All patients who responded to avatrombopag maintained response for 88.7% of their time on treatment. Patients who responded without the need for rescue therapy maintained their response for 93.6% of their time on avatrombopag.
Patients who switched for convenience maintained a response for 96.5% of the time on avatrombopag. Patients who switched for adverse events maintained a response for 90.2% of the time. Patients who switched for efficacy maintained a response for 68.2% of the time.
Overall, the median platelet count for all avatrombopag exposure was 107×10 9/L. The median platelet count for convenience switchers was 129×10 9/L, efficacy switchers was 60×10 9/L, and adverse event switchers was 93×10 9/L.
CONCLUSION: In a heavily pretreated chronic ITP population who switched from another TPO-RA to avatrombopag, the initial response to avatrombopag was both durable (with up to 74% of patients never experiencing a loss of response) and stable (with patients maintaining a response on average for up to 89% of the time).
Al-Samkari: Argenx: Consultancy; Dova/Sobi: Consultancy, Research Funding; Novartis: Consultancy; Amgen: Research Funding; Rigel: Consultancy; Agios: Consultancy, Research Funding; Moderna: Consultancy. Gernsheimer: Principia: Research Funding; Rigel: Research Funding; Amgen: Honoraria; Novartis: Honoraria; Cellphire: Consultancy; Dova: Consultancy; Sanofi: Consultancy. Liebman: Pfizer: Consultancy; Dova: Consultancy, Honoraria; Argenx: Research Funding; Amgen: Consultancy; Sanofi/Genzyme: Research Funding; Novartis: Consultancy, Research Funding. Lee: Dova: Honoraria. Bernheisel: Sobi, Inc.: Current Employment. Kolodny: Sobi, Inc.: Current Employment. Wojdyla: Sobi, Inc.: Current Employment. Vredenburg: Sobi, Inc.: Current Employment. Jamieson: Sobi, Inc.: Current Employment. Cuker: Takeda: Research Funding; Sanofi: Research Funding; Spark Therapeutics: Research Funding; Pfizer: Research Funding; Novo Nordisk: Research Funding; Novartis: Research Funding; Bayer: Research Funding; Alexion: Research Funding; UpToDate: Patents & Royalties; Synergy: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal